BACKGROUND: Relapsed/refractory (R/R) acute leukemia patients ineligible for allo-HSCT have poor prognosis. CAR-T therapy has shown promising therapeutic outcomes for these patients, but simultaneously confers the risk of pancytopenia and potential tumor relapse. Bridging CAR-T therapy with allo-HSCT has the potential to restore hematopoiesis and consolidate long-term efficacy. However, GvHD remains a major impediment, and the myeloablative conditioning regimen eradicates residual CAR-T cells and compromises CAR-mediated antitumor function. Furthermore, the implementation of conditioning regimen and GvHD-prophylaxis could lead to severe toxicities. Novel strategies are therefore warranted to preserve CAR-T cells, enhance antitumor potency and minimize complications.
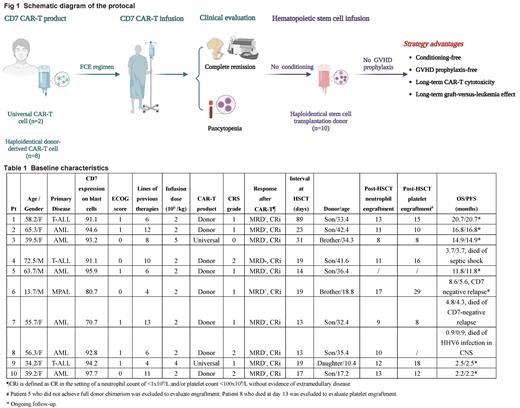
METHODS: In this study, we presented 10 patients (enrolled between November 2021 and June 2023) with R/R CD7-positive acute leukemia who proceeded directly to haploidentical HSCT (haplo-HSCT) after CAR-T therapy. All patients received allogeneic CD7 CAR-T therapy, manufactured from either haploidentical donors (n=8) in a phase I clinical trial of donor-derived CAR-T cells (NCT04599556) or (n=1) universal CD7 CAR-T cells (RD13-02) therapy (NCT05716113) or as a compassionate use program after the end of a phase I clinical trial of universal CD7 CAR-T cells (RD13-01) (NCT04538599). CAR-T cell doses were at 2´10 6(haploidentical donor, n=8), 4´10 6 (RD13-02, n=1) or 5´10 6 (RD13-01, n=1) cells/kg. After achieving complete remission with incomplete hematological recovery (CRi), all patients underwent haplo-HSCT without conditioning regimen or GvHD prophylaxis. The primary end point was efficacy, safety, hematopoiesis recovery, and donor chimerism.
RESULTS: All patients relapsed from multiple lines of chemotherapy or immunotherapy (median, 7; range, 4-13), were ineligible for allo-HSCT and received palliative care. After CAR-T infusion, 9 patients achieved MRD-negative CRi, and 1 achieved MRD-positive CRi, on day 15 after CD7 CAR-T therapy. 9 patients developed grade 1-2 cytokine release syndrome, and no neurological toxicity was reported. All patients exhibited grade 4 pancytopenia and bone marrow hypocellularity and were directly bridging to haplo-HSCT. The median time from CAR-T infusion to haplo-HSCT was 19 (range, 15-89) days. One patient died of HHV6 infection in central nervous system on day 13 after haplo-HSCT and was not evaluable for the following safety and efficacy. 3 patients experienced short-term grade II acute GvHD, which was resolved completely after low dose steroid, anti-TNF-α or ruxolitinib therapy. One patient developed PTLD at 2.1 months post-HSCT. In 9 evaluable patients, 8 showed full donor chimerism at 1-month post-HSCT, and 1 showed autologous hematopoiesis recovery. At a median follow-up of 10.2 (range, 2.2-20.7) months after CAR-T therapy, 6 patients remained in MRD-negative CR with normal hematopoiesis (including the patient with autologous hematopoietic recovery), 1 had CD7-negative relapse at 5.6 months, 1 developed CD7-negative relapse at 4.3 months and died at 4.8 months, and 1 died from septic shocks at 3.7 months. OS, PFS, NRM, and CIR at 1 year were 64.3% (95% CI, 38.5%-100%), 51.4% (95% CI, 26.2%-100%), 22.9% and 25.7%, respectively. Peripheral normal T cells in 8/10 of patients remained CD7-negative at the last follow-up, illustrating a well-maintained CAR-T function post-HSCT.
CONCLUSIONS: Our findings demonstrate a novel strategy to simultaneously achieve CAR-T persistence and complete HSC engraftment, while reducing the risks of GvHD, providing valuable insights into combining cellular therapy with allo-HSCT for the treatment of R/R acute leukemia patients ineligible for conventional allo-HSCT.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal